MDR certificate for the NeoDoppler in two years
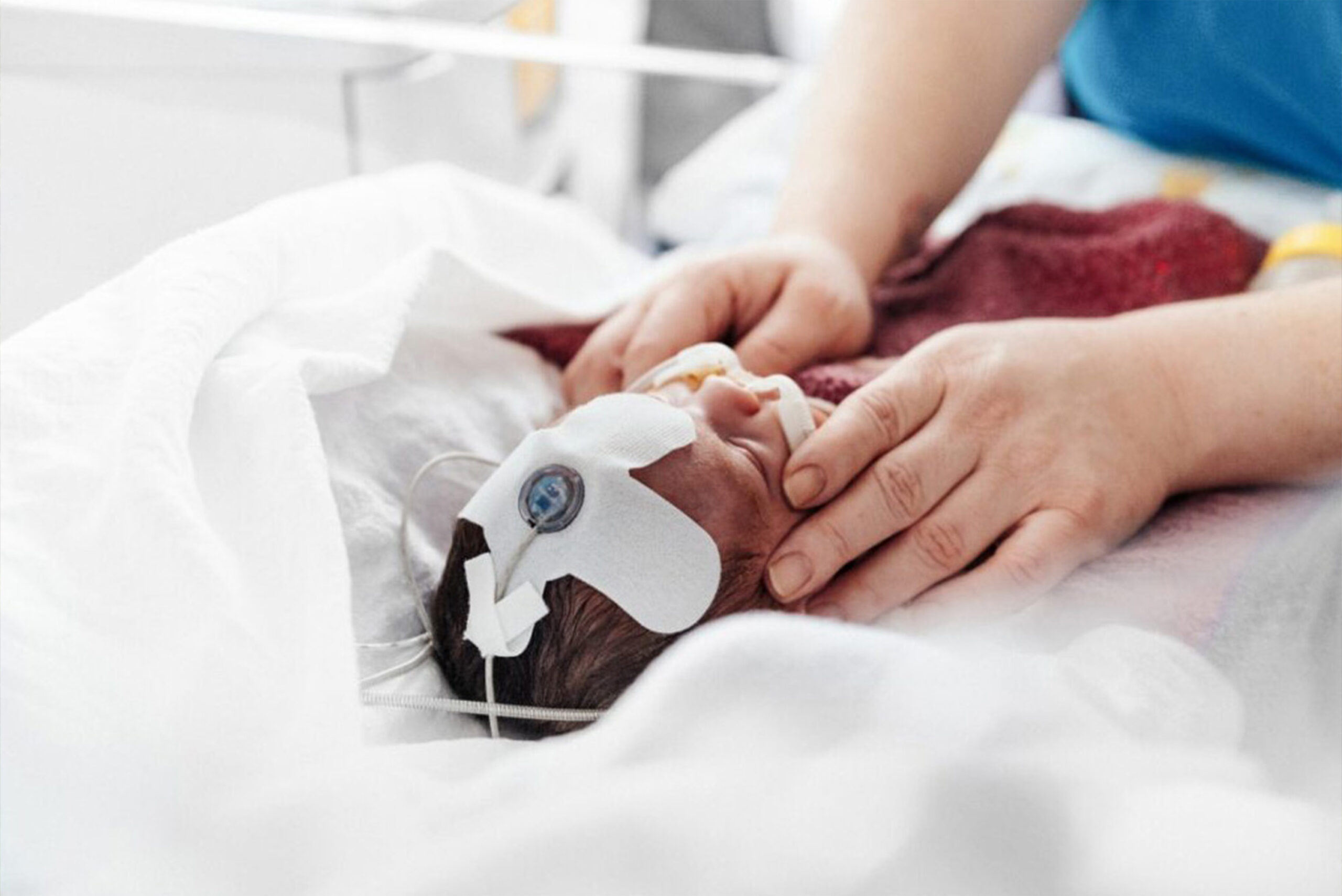
Continuous monitoring of blood flow in the brains of premature babies and infants can save their lives. This is because around 10 percent of all newborns are premature babies, who are exposed to an increased risk of brain damage in the first few months of life. Despite the need for early care, no specialized ultrasound equipment has been available on the market – until now. With the vision of creating life-saving solutions, the Norwegian start-up Cimon Medical has developed the NeoDoppler as the first non-invasive ultrasound device of its kind for premature babies – together with BAYOOMED and BAYOOCARE.
When the Cimon Medical team came to the BAYOOMED and BAYOOCARE stand at MEDICA in November 2019, a prototype had already been developed. What was missing was the right hardware and software that would merge the algorithm developed by Cimon with the medical device and thus realize the idea.
From getting to know each other to the start of the NeoDoppler project
Just six weeks after the pitch, the project teams met in Norway for a workshop to get to know each other and clarify all stakeholder requirements. In addition to the development of hardware and software by BAYOOMED, BAYOOCARE was to assume responsibility as legal manufacturer for the NeoDoppler.
The project was finally launched a few months later. The main challenge in 2020 was to develop the product in accordance with the applicable Medical Device Regulation (MDR) for risk class IIb.

“That’s why we contacted the notified body very early on, two years before the NeoDoppler was launched on the market, and started the conformity assessment procedure.”
Alfred Koch, CEO of BAYOOCARE
The stack: medical software behind the medical device
On a technical level, the BAYOOMED team developed a highly specialized software stack for the medical device, which, among other things, links and evaluates the device’s probe signals based on C++ code. The algorithm developed by Cimon Medical was re-implemented for use and thus adapted to the target system of the NeoDoppler.
“For us, it was the first project in which we took responsibility for both the software and the hardware,” says Bernd Seidenspinner, CTO of BAYOOMED. “We initially created the UI on a web basis. However, this did not meet our requirements, so we switched to QT.” The central technical element of the NeoDoppler is also an MQTT broker – external software that sends messages between individual modules and components, thereby improving functionality.
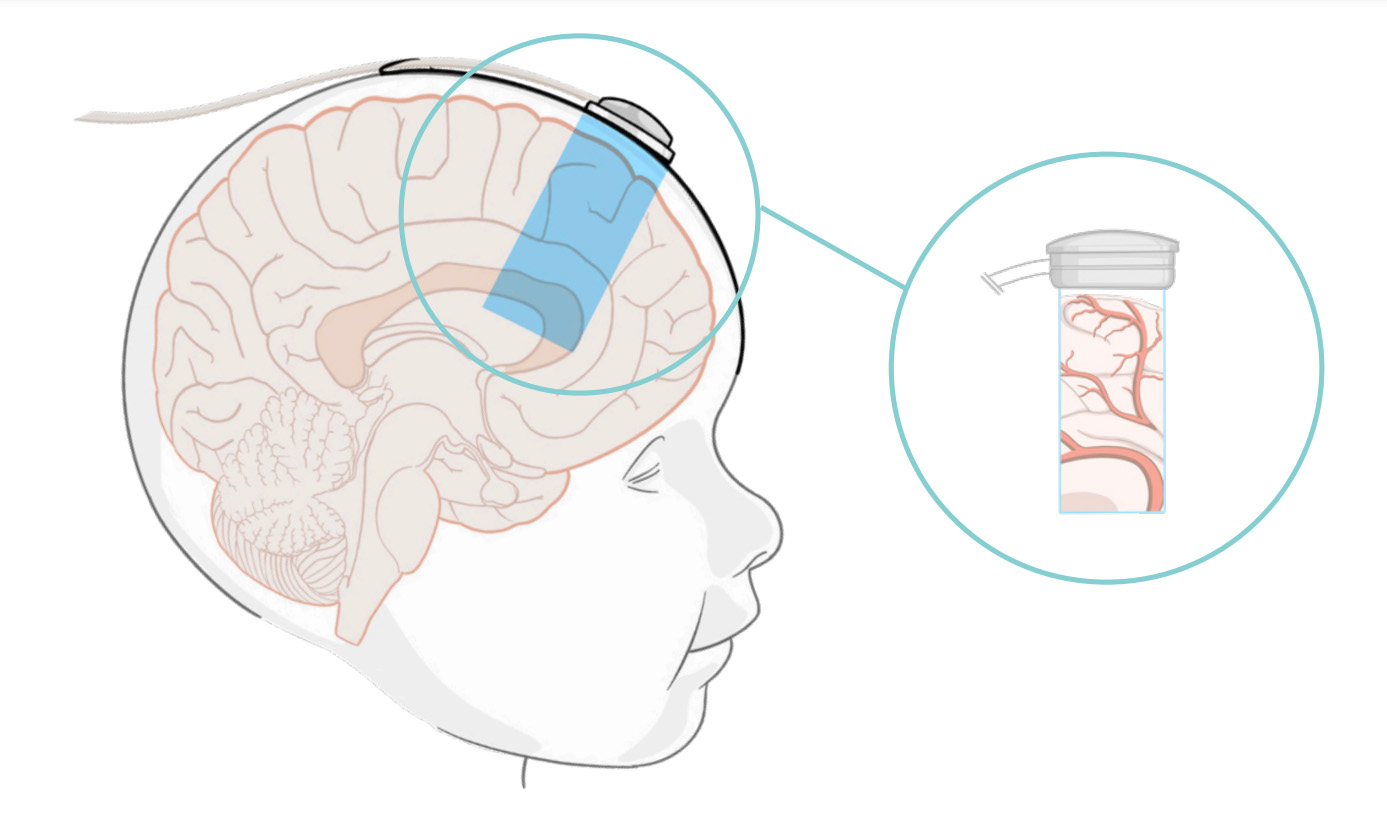
How the NeoDoppler works
NeoDoppler compact: The ultrasound device offers these advantages
With the NeoDoppler, Cimon Medical and BAYOOMED have developed the first hemodynamic monitoring system for premature babies. For example, changes in blood flow in the brain of premature babies can be detected immediately, allowing nursing staff to intervene quickly. In addition, no specialized knowledge or a thorough introduction is required to operate the system.
Blood flow is measured directly and continuously, a major advantage compared to other ultrasound devices, which often measure indirectly or with interruptions. The flexible system is also suitable for the integration of third-party systems.

“Developing the NeoDoppler as the first ultrasound device of its kind and with all these advantages that could save the life of a premature baby – that was a special task and honor for us.”
Bernd Seidenspinner, CTO of BAYOOMED
CE marking and MDR certificate in two years
Two years passed between the first meeting and the CE marking of the NeoDoppler. “Of course, challenges also arose during the project. For example, we had to adapt our insulation strategy,” says Alfred Koch. “But because the project teams worked together excellently, from communication to solving challenges, we were able to complete the project in record time.” The NeoDoppler 2022 was one of the first thousand medical devices to receive an MDR certificate.
To date, the NeoDoppler from Cimon Medical has been available for testing in neonatal intensive care units in numerous university hospitals across Europe. The BAYOOMED team is also continuously working on change requests to further optimize the medical device.