Your platform: Get to know MedicalOne Connect
Your platform: Get to know MedicalOne Connect
Connect healthcare professionals, patients, medical device manufacturers, the pharmaceutical industry, life sciences and, last but not least, payers and insurance companies – all with one platform.
In compliance with GDPR, MedicalOne Connect optimizes communication between all actors in a secure and cost-efficient manner. The DTx platform offers personalized treatment plans and tracks their progress. Technologies such as AI and machine learning are leveraged to enhance the effectiveness of healthcare programs, ultimately leading to improved patient care.
Your benefits
Accelerated market introduction
Cost savings of up to 60%
Pre-validated
MDR and FDA compliant
GDPR and HIPAA compliant
BSI TR-03161 compliant
BSI TR-03161 compliant
Security by design
Security by design
Scalable design
State-of-the-Art Tech-Stack
Flexibly expandable
How the MedicalOne Connect DTx platform works
Moreover, the MedicalOne Connect DTx platform empowers healthcare stakeholders to create customized apps for their patients or clients. These digital companions may include various elements such as a diary (for medication, exercise, nutrition), standardized QoL questionnaires, news feeds, and educational content (articles, videos, training guides) to assist individuals in achieving their health goals. The platform serves as an ideal foundation for connecting with external devices like fitness trackers via BLE and interoperability by supporting the FHIR standard.
A personalized backend offers dashboards, data visualization, exports, and reports for various use cases, including Real-World Evidence studies or patient monitoring.
Components of MedicalOne Connect DTx: The Skeleton App
Our cross-platform Skeleton app: It offers all the standard functions that a companion app needs. This includes DTx for registration & login, profile & settings and onboarding options. Users of the app can also keep a diary and benefit from educational content and a newsfeed. The BLE communication interface is preset.
Customizable
We adapt the Skeleton App to your corporate design or branding – completely individually. We also develop a toolkit for workflows as well as content and additional functions according to your needs.
Connectable
Whether medical device, smartwatch, fitness tracker or wearable – the Skeleton app can be easily connected to various medical devices. This allows data to be synchronized easily.
User Tested
Because usability is one of the most important factors in medical apps, we not only pre-validated the skeleton app, but also successfully tested it in human factors studies.
The platform in the cloud
Devices like fitness trackers, smartwatches, or apps (DiPA, DiGA) store a wealth of data. With MedicalOne Connect DTx, this data can be collected in a cloud to generate reports and facilitate collaboration.
These product packages await you
We offer three product packages tailored to your individual needs. Benefit from our packages that combine several services at once. How can our platform support you in implementing your digital health innovation?
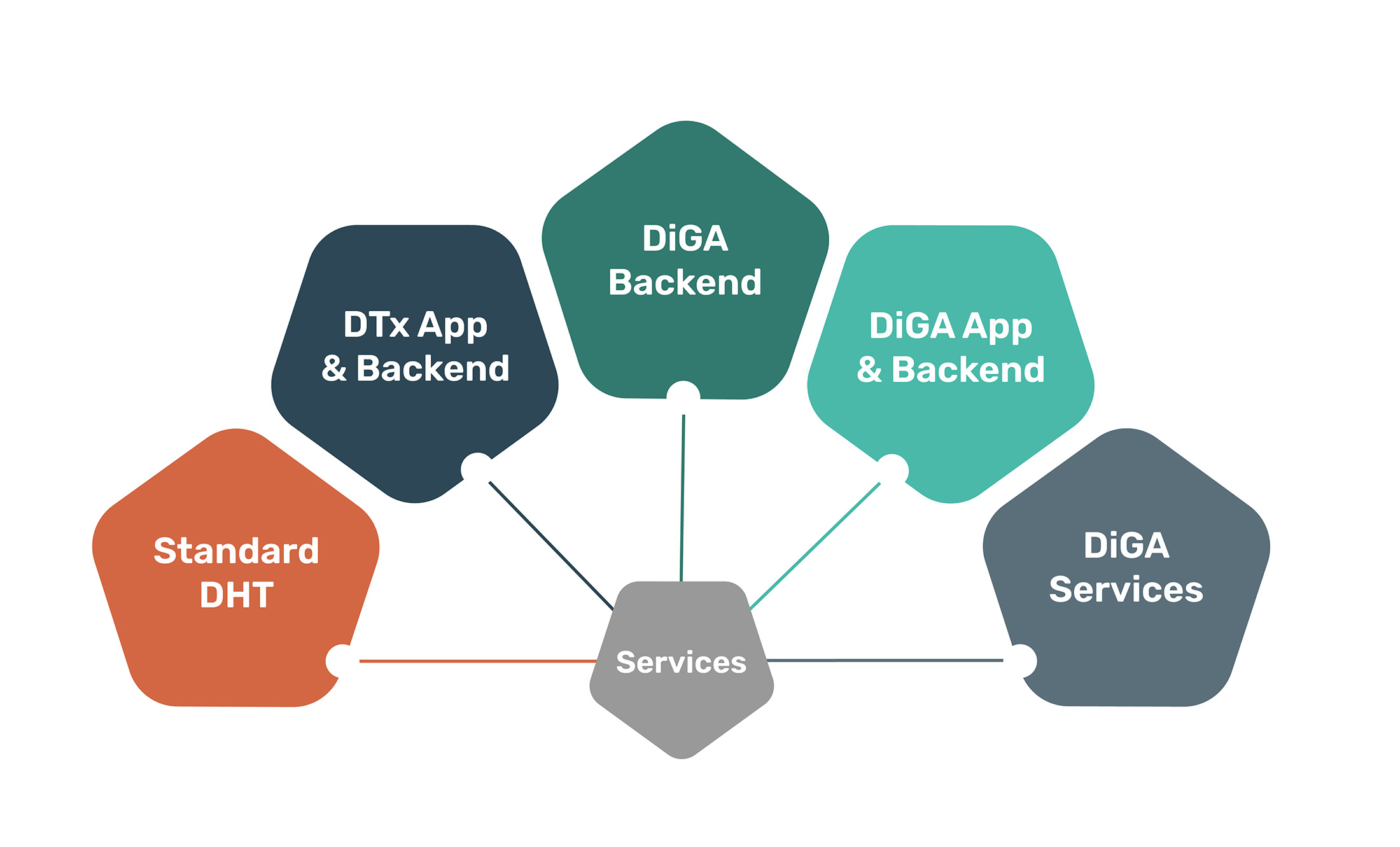
Standard DTx
App & Backend
App & Backend
General features:
- Onboarding, including consent management for Terms and Privacy
- Standard profile and settings
- Identity and access management with OpenID Connect Standard
- Diary and dashboard
- 5 reminders and a general notification framework
- 1 standard BLE connection
- 10 articles with educational content (text or video)
- Standard imprint, error logging, operating system compatibility test
In-app reporting & 1 PDF export - Encryption (at rest state and transmission)
- Simple user tracking
- Cloud for data storage and services
Including:
Technical documentation for class IIa medical device in accordance with the Medical Device Regulation (MDR), staging and production system
Standard DiGA
App & Backend
App & Backend
Including all standard features of the standard DTx version.
Features:
- ePA (Electronic Health Record)
- Health ID
- Code validation
- Invoicing
- Interoperability (MIO – Medical Informatics Object)
- Additional prescription reminders
- Data import to bridge prescription gaps
- Internal penetration testing
DiGA
Services
Services
Features:
- ePA (Electronic Health Record)
- Health ID
- Code validation
Invoicing - Interoperability (Export & MIO Mapping)
Specifications are provided to communicate with the microservices.
Additional customizing & services on request
Adaptation of the existing application for the use of the microservice architecture
DiGA
Backend
Backend
- Compliant with BSI TR-03161 / DiGA data protection criteria (BfArM)
- Concept for data deletion for our backend services
Features:
- Authentication service / IDP
- Audit and Inbox Service
- Support and Helpdesk Web Interface
- Notification and tracking service
- Consent Service
- App data storage in the backend
- Export service (customer data and connection to ePA and MIO)
- Status monitoring and warnings in the event of errors
Your BSI TR-03161 compliant backend in 2 months
Including:
Staging and test system, migration of specific business logic if required, consulting and support with certification and customization of the app
The package includes up to 3 days of consulting to customize the interfaces between the app and the backend.
Looking for something personalized?
We also develop a CustomizedDTx (app & backend) for you – tailored precisely to your needs.
Additional modules from the toolkit or customizing and services are available on request. Benefit from:
- Customized UI and workflows
- CRM for product management
- Study backend, customized dashboards, and data dumps
- Additional and customized BLE services
- Different user tracking technology
- Push notifications
- Extended penetration testing
- E2E encryption and customized cloud hosting
- Compliance assessment / FDA approval support
- Legal manufacturer as a service
Included here is also the technical documentation for a medical device class IIa in accordance with Medical Device Regulation (MDR), as well as staging and production systems.

