From the idea to post-market surveillance of your DiGA
The life cycle of a digital health application (DiGA) has various stages, as with any medical device. However, the development of a DiGA also requires knowledge of the best way to achieve reimbursability and the requirements that need to be met for listing in the DiGA directory, among other things.
Would you like to launch a DiGA on the market, but still need the right partner at your side who knows all the tricks and tips? Then you’ve come to the right place, because we can realize your project with the combined expertise of our partners.
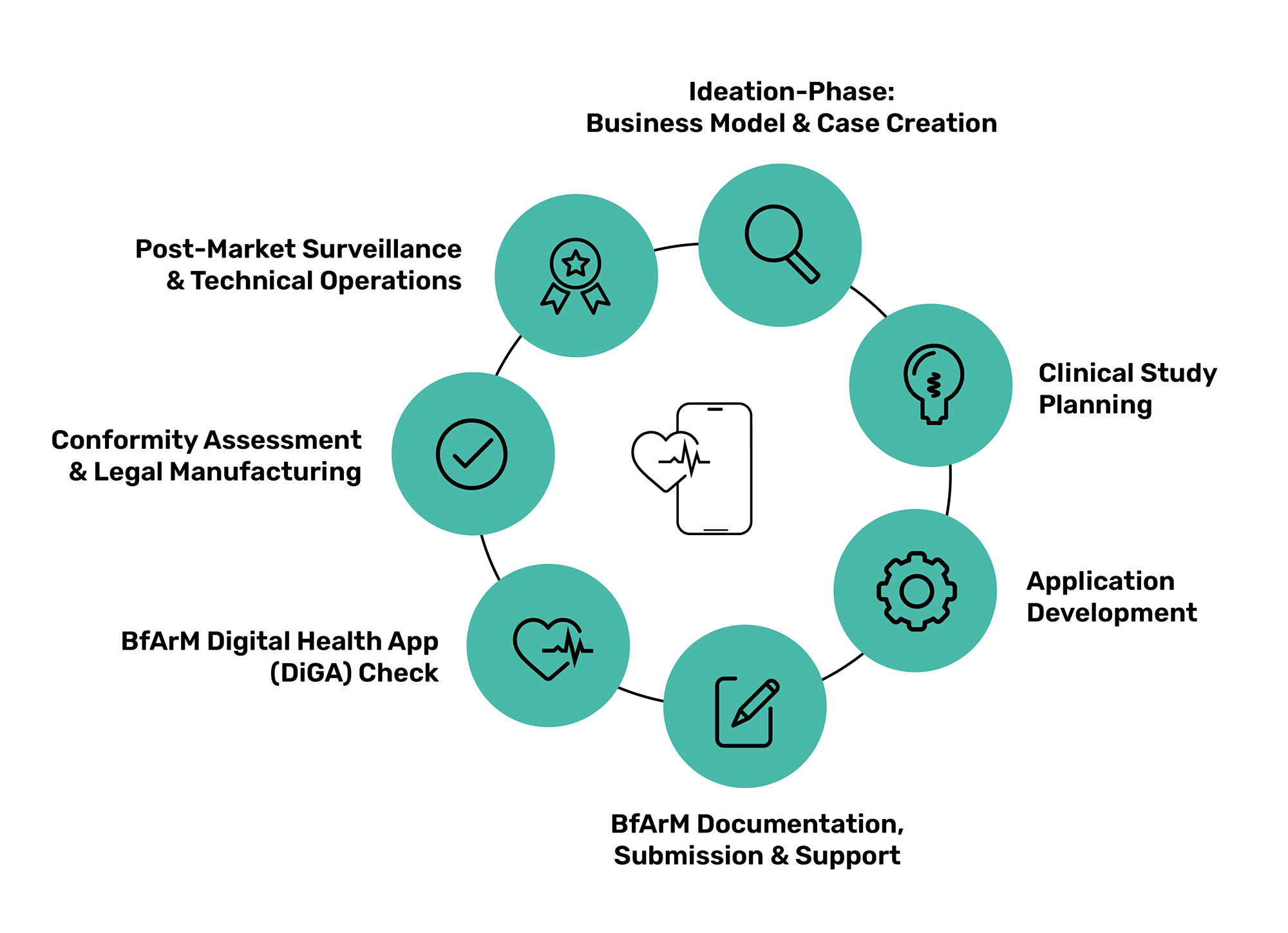

Business Model & Case Creation and BfArM DiGA-Check
Together with one of our partners, we will design an initial reimbursement concept for you with possible paths to reimbursement in the German healthcare system. We will provide you with comprehensive advice on your specific DiGA use case and possible supply effects. We will also support you in discussions with the Federal Ministry for Drugs and Medical Devices (BfArM).
Clinical Study Planning
Our partner will support you in defining the therapeutic benefit, selecting the positive care effects and designing and planning your clinical trial. With their extensive experience from several successfully listed DiGAs, they are a reliable and proven partner who will work with you to make your DiGA a success.
Application Development
Whether with our MedicalOne platform as the basis for your DiGA or a complete individual development – we support you in the development of your product. We not only produce the software, but can also take care of all the technical documentation, including cybersecurity assessments, risk management and requirements engineering.
We also offer interfaces for connecting your DiGA to the telematics infrastructure. This ensures that you always meet the current requirements of the BfArM and the DiGAV.
Together with our partners for invoicing and code validation as well as for access to the TI, we provide a one-stop solution that you can rely on.
BfArM Documentation, Submission and Support
Our partner will help you to apply for a consultation with the BfArM and prepare it with maximum benefit for you. They will accompany you during the consultation with the BfARM and support you in preparing and submitting the DiGA application. Our partners will also navigate you through the entire DiGA fast-track process.
Conformity Assessment & Legal Manufacturing
Together with BAYOOCARE – our YOOme partner – we support you both as a legal manufacturer and with conformity assessment. BAYOOCARE assumes product liability as a legal manufacturer for products of all classes. They check the documentation of the product based on the requirements of the Medical Device Regulation (MDR) and support you during audits.
BAYOOCARE has a 27001 certificate and is therefore also available as a legal manufacturer for DiGA.
Post Market Surveillance & Technical Operations
We are also at your side after the product has been placed on the market: both for the operation of the application, including ensuring that your software is up to date, as well as for support for your product. We also accompany you when it comes to carrying out post-market surveillance.

